Image Processing Allows Reliable In Vivo Detection and Quantification of Retinal Microglia Following Laser-Induced Choroidal Neovascularization
Abstract
Purpose
Macrophage and microglia cells have proved to have an important role in pathological neovascularization conditions such as age-related macular degeneration. The pathomechanisms that trigger microglia activation remain still unknown though. New in vivo approaches are necessary for a better understanding and evaluation of the microglial behavior, all combined with image enhancing techniques.
Methods
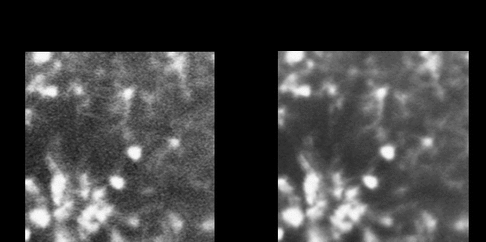
We used MacGreen mice, expressing EGFP protein in macrophages and trophoblast cells. An argon ion laser was used to induce choroidal neovascularization (CNV). Laser scars were held only in half of the eye, allowing in vivo spatial and time-response behavior studies by scanning laser ophthalmoscopy (SLO) in the autofluorescence mode for the fundus (FAF). In vivo recording was processed by different algorithms to reduce noise, remove moving elements (flowing macrophages) and increase shape definition. Data was verified ex vivo by retinal sections and whole-mount preparations.
Results
Two different populations of microglia were detected using FAF: in the inner and outer plexiform layers (IPL and OPL) and subsequently verified ex vivo. We chose the algorithm based on frame averaging as the most appropriate to enhance images for further analysis, enabling quantification based on morphology criteria (see figure below). Microglia activation was studied by recognizing amoeboid-like cells after the laser procedure. Time-dependent activation changes were found in the IPL at D1, with a peak at D4 and a decrease and maintenance thereafter. In terms of spatial activation, we described a progressive spreading of amoeboid cells from the laser scar to overall the retina in the IPL. No significant time-spatial changes were observed in the OPL. When the laser power was increased it was not correlated with stronger microglia activation.
Conclusions
SLO results an excellent tool for in vivo imaging when combined with computational enhancement. MacGreen mice have been shown to be also an appropriate model for studying time and spatial dependent microglia activation. As CNV is performed by damaging the outer retina, it is surprising to find a major activation at the IPL, shedding light on new perspectives and establishing a model that eases translational purposes.